Self-Driving Labs
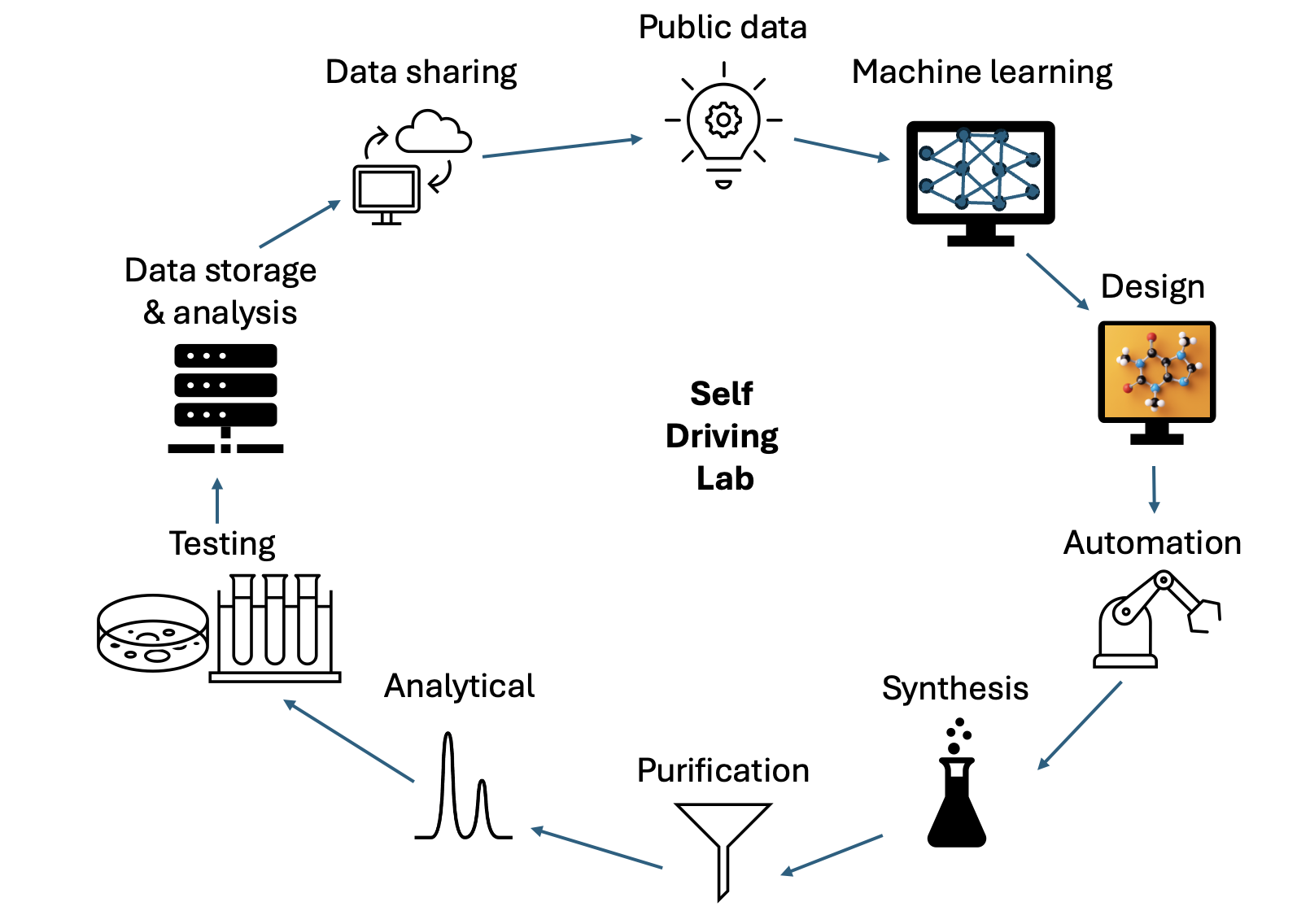
Our software can be used as a key part of the design-make-test-analyze cycle.

Our software can also be integrated into self driving labs where we could leverage public data and data generated in the lab to develop machine learning models that could then enable the design of new molecules prior to their synthesis and testing.
Rare Disease Registry Development

A patient registry is a place to store detailed information about patients with a specific disease or syndrome potentially allowing the collection of ‘real world data’. Guidance is available from the FDA, EMA and others on the building and use of registries for supporting regulatory decision making for drugs and biological products (See references below).
Through the necessity of needing a rare disease registry we have created our own for one of the rare diseases we work on in order to help us find patients for a future clinical trial. The registry was created in Python using the Django web framework. All data collected is stored in a SQLite database file that can be used to retrieve information that has been stored on the site. Over the coming months we will be seeking IRB approval and launching this registry, sharing more details here on our progress.
We realize this may also be a useful capability for others to learn from our expertise. If you would like a rare disease registry custom built perhaps we can assist you in your journey too. Please contact us for further details!
Pisa F, Arias A, Bratton E, Salas M, Sultana J. Real world data for rare diseases research: The beginner’s guide to registries. Expert Opinion on Orphan Drugs. 2023;11(1):9-15. doi: 10.1080/21678707.2023.2241347.
Wicks P, Wahlstrom-Edwards L, Fillingham S, Downing A, Davies EH. So You Want to Build Your Disease's First Online Patient Registry: An Educational Guide for Patient Organizations Based on US and European Experience. Patient. 2023;16(3):183-99. Epub 2023/03/23. doi: 10.1007/s40271-023-00619-w. PubMed PMID: 36947286; PMCID: PMC10031688
Agency EM. Guideline on registry‐based studies 2023. Available from: https://www.fda.gov/media/154449/download.
Mordenti M, Boarini M, D'Alessandro F, Pedrini E, Locatelli M, Sangiorgi L. Remodeling an existing rare disease registry to be used in regulatory context: Lessons learned and recommendations. Front Pharmacol. 2022;13:966081. Epub 2022/10/11. doi: 10.3389/fphar.2022.966081. PubMed PMID: 36210847; PMCID: PMC9537464.
Kolker S, Gleich F, Mutze U, Opladen T. Rare Disease Registries Are Key to Evidence-Based Personalized Medicine: Highlighting the European Experience. Front Endocrinol (Lausanne). 2022;13:832063. Epub 2022/03/24. doi: 10.3389/fendo.2022.832063. PubMed PMID: 35317224; PMCID: PMC8934440.
Hageman IC, van Rooij I, de Blaauw I, Trajanovska M, King SK. A systematic overview of rare disease patient registries: challenges in design, quality management, and maintenance. Orphanet J Rare Dis. 2023;18(1):106. Epub 2023/05/06. doi: 10.1186/s13023-023-02719-0. PubMed PMID: 37147718; PMCID: PMC10163740.
Sames L, Moore A, Arnold R, Ekins S. Recommendations to enable drug development for inherited neuropathies: Charcot-Marie-Tooth and Giant Axonal Neuropathy. F1000Res. 2014;3:83. Epub 2014/05/27. doi: 10.12688/f1000research.3751.2. PubMed PMID: 24860645; PMCID: PMC4023663
